Dry Fog 2S
Dry Fog 2 is a high-tech, easy-to-use solution for the biodecontamination of cleanrooms and other critical environments.
Intrafluid has an effective Dry Fog 2S system with a unique and safe system.
About Dry Fog 2S
Designed to be used with Peracetic Acid concentrate formulations, the combination of a highly effective biocide and our state-of-the-art Dry Fog delivery system enables users to rapidly and safely deliver peracetic acid and hydrogen peroxide vapours to even the most complex areas.
Its sanitary and 100% autoclavable design allows it to be used in the most critical areas of the pharmaceutical and other industries where maintaining the highest level of sterility is a priority.
Standard Features & Benefits
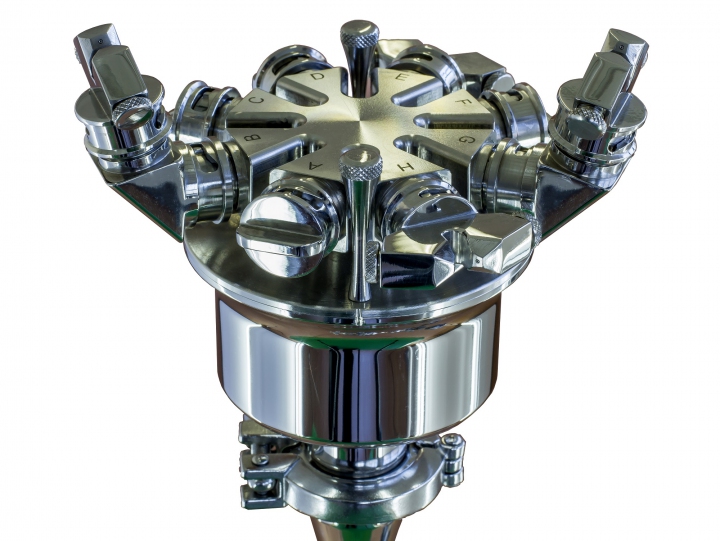
Configurable Spray Head for multiple large areas
Newly designed spray apparatus allows for simultaneously up to eight different nozzle(s) positions increasing the coverage possibilities for higher flexibility inside volumes up to 1.000 cubic meters (35,000 ft3) including multiple rooms areas. In addition, the Dry Fog 2S head enables horizontal and/or vertical direction spray diffusion.
Short Process Time
Typically, the entire bio-decontamination process can be completed in less than 3 hours, depending upon room size and ventilation system efficiency, compared to up to tens of hours with some conventional systems.
- Substantially lower cleanroom downtime.
- The diffusion and contact times are optimized.
- The venting time is minimized due to the very low quantity of chemistry vaporized in the air.
Optimal Efficacy with Concentrate Peracetic Acid Formulations
Less than 2ml of 5% peracetic acid formulation per cubic meter of room is diluted in purified water and spayed out the systems.
This procedure has been validated by hundreds of pharmaceutical companies over the world and the Dry Fog 2S’s efficacy against bacteria, yeast, mould, bacteria spores and viruses is conforming to EN 17272 (April 2020) for Industrial and Healthcare applications.
No corrosion issue have been reported by hundreds of users in pharmaceutical modern clean room environment.
Dry Fog 2S can also be used with Hydrogen Peroxide formulations.
Highly Flexible System
The system is easily adaptable to differing room dimensions and configurations and can be adjusted to suit any room height.
- Robust construction with no moving part and almost zero maintenance.
- No electronic part and does not require electrical connection.
- Sanitary 100% autoclavable design using 316L Stainless Steel and Titanium for key components.
- Coverage of up to 1.000 m3 (35,000 ft3) with one single unit.
- One step Bio-Decontamination of multiple rooms area.
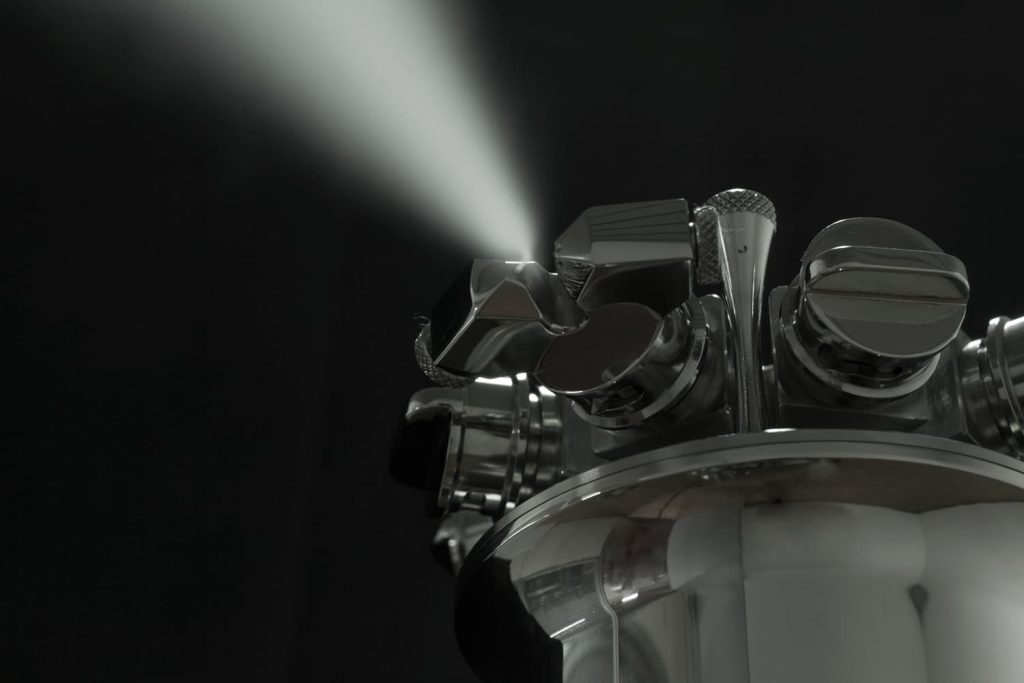
Ultrafine Droplets
The Dry Fog 2S equipment is designed to produce ultrafine atomized droplets which will ensure even dispersion of the disinfectant or biocide chemical vapours throughout the room.
- Controlled and consistently accurate droplet size.
- Minimized risk of condensation.
- Ensures distribution into normally inaccessible areas.
Very Hight Reproducibility
Dry Fog procedure has been developed in Europe about 20 years ago together with and for pharma companies. It is conforming to pharmaceutical needs and offers a very high level of reproducibility on the efficacy of course but also on the system’s positioning and configuration.
The vapours dispersion into the room(s), the contact times as well as the venting times at strategic locations can be very simply measured, monitored and documented in accordance with pharmaceutical requirements.
Every detail has been designed to help on a very detailed SOP documentation and a perfect reproducibility at each bio-decontamination.
Quality, Documentation and Qualification
100% of the Dry Fog 2S manufacturing is tested out of production. Every critical part in contact with the biocide is identified, serial numbered and officially certified conforming to specifications.
Dozens of specific certificates are supplied with each System. An optional IQ-OQ-PQ Qualification package is available.
Technical data
Product specifications:
Dry Fog machine dimensions: 42 cm x 33 cm x 183 cm.
Shipping dimensions: 50 cm x 50 cm x 120 cm.
Dry Fog machine weight: 28 Kg.
Shipping weight: 35-50 Kg.
Compressed air supply: 5 bar minimum 70 L/min spray nozzle air supply.